

Explore The Possibility of
Changing Your Classic CAH Journey
Available Trial Sites

Australia
| Site/Institution | Contact Information | |
| Royal Melbourne Hospital (City Campus), Victoria 300 Grattan Street, Parkville Victoria 3050 | Bina Patel Diabetes Research Nurse/study Co-ordinator Department of Diabetes and Endocrinology The Royal Melbourne Hospital – City Campus, Level 4 West, RM424 Ph: + 61( 3) 9342 7672 Email: bina.patel@mh.org.au Working Days: Mon, Tues and Wed | |
| Blacktown Hospital, NSW 18 Blacktown Rd, Blacktown NSW 2148 | Allison Sigmund Clinical Research Support Manager Clinical Research Support Unit Ph: +61(2)8890 4336 / Mob: 0499 941 490 Email: allison.sigmund@health.nsw.gov.au | |
| Keogh Institute for Medical Research, WA Hospital Avenue, Nedlands WA 6009 | Smilja Dragovic Clinical Trial Coordinator Workdays: Monday-Thursday Keogh Institute for Medical Research QEII Medical Centre, C Block 1st Floor Ph: +61(8) 6457 2475 Email: smilja@kimr.org | |
| Royal Prince Alfred Hospital, NSW John Hopkins Dr, Camperdown NSW 2006 | Nick Fuller Senior Research Fellow Level 2 Charles Perkins Centre D17 Ph: +61(2) 8627 1932 Email: nick.fuller@sydney.edu.au | |
| Royal Brisbane and Women’s Hospital, QLD Butterfield Street, Herston QLD 4029 | Helen Legg Study Coordinator Department of Endocrinology and Diabetes Level1 Dr James Mayne Building Ph: +61 (7) 36465155 / Mob: 0412795200 Email: helen.legg@health.qld.gov.au | |
| Lyell McEwin Hospital, SA Haydown Rd, Elizabeth Vale SA 5112 | Brenda Trezona Clinical Research Coordinator Clinical Trials Unit Lyell McEwin Hospital, Level 2 Ph: +61 (8) 82820666 / Mob: 0414 542394 Email: brenda.trezona@sa.gov.au | |

Before a medication can be prescribed by a health care provider, it must be tested. Clinical trial programs are health-related research studies in humans that follow a pre-defined, detailed plan to determine the safety and effectiveness of the investigational medication for its intended use.
The primary purpose of the CAHmelia program is to assess if tildacerfont is effective in lowering androgens (testosterone-related hormones) and daily glucocorticoid doses in adults with classic CAH. The CAHmelia studies are dedicated to exploring solutions for people living with classic CAH.(4,5)
Living with classic Congenital Adrenal Hyperplasia
Classic Congenital Adrenal Hyperplasia (CAH) is a rare genetic condition that affects the body’s ability to create and regulate key hormones. Most significantly, CAH alters the production of cortisol, which is known as the “stress hormone.” CAH also affects the production of aldosterone, which regulates salt and water balance in your body, and androgens which are testosterone-related hormones present in men and women.(1)
Managing CAH
Do you live with classic Congenital Adrenal Hyperplasia (CAH)?
Currently, glucocorticoid (GC) therapy is the only approved treatment for classic CAH. GCs are a type of steroid treatment that can help you manage your condition by replacing deficient cortisol and reducing androgen levels.(2)
Replacing cortisol with steroids is necessary to maintain health in people with classic CAH. However, many people with classic CAH also need steroids to decrease their androgen production to control symptoms such as excess body hair, fertility challenges, irregular menstrual periods, and testicular adrenal rest tumors (TARTS).(2)
Steroid therapy goals are to prevent life-threatening adrenal crisis across all ages, provide balanced hormone levels and promote normal growth and development.
To learn more about the condition of classic Congenital Adrenal Hyperplasia (CAH), watch these videos with SpruceBio Chief Medical Officer and Endocrinologist, Dr. Will Charlton.
Meet SpruceBio Chief Medical Officer, Dr. Will Charlton as he discusses classic Congenital Adrenal Hyperplasia (CAH) and it’s effects on patients.
Glucocorticoid related signs and symptoms to W.A.T.C.H.
Weight Gain • Appetite lncrease • Temperament/Mood Changes • Circular or Moon Face • Hair Growth & Acne (facial)
Your body may be sending you a message about hormone imbalance from glucocorticoid (steroid) under-treatment or over-treatment.(1)

A survey of 113 CAH participants stated that they do not feel sufficiently informed about their treatment:*

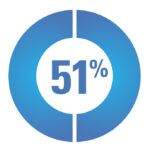
51% of participants felt they did not have enough access to information to make an informed choice about GC treatment
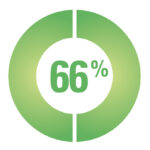
66% of participants are willing to change their current regimen if they could lower their dose of steroid
*Data on file Spruce Bio Participant Survey 2022
A survey of 113 CAH participants reported that side-effects are common*
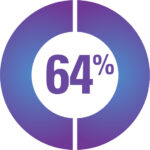
64% Weight Gain
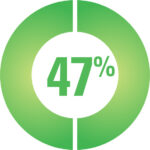
47% Fatigue
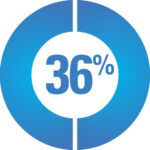
36% Mood Swings
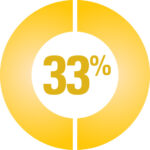
33% Depression
*Data on file Spruce Bio Participant Survey 2022
What is tildacerfont?
Tildacerfont is a new type of oral, once-daily investigational drug that is NOT a steroid.(3) By reducing the amount of androgens (testosterone-related hormones) your body makes, tildacerfont may improve your classic CAH symptoms.(3) This investigational drug will not replace your steroid treatment but may allow you to manage your condition with lower amounts of steroids.
Is tildacerfont safe?
Tildacerfont is generally well-tolerated in healthy volunteers and in people with classic CAH:
- Generally well-tolerated at doses under evaluation
- Generally well-tolerated across a diverse group of people
Tildacerfont is generally well-tolerated in healthy volunteers and people with classic CAH. Tildacerfont’s global history includes 8 studies with 230 participants, in 92 centers, and 20 countries.

Who can take part in this Study?
You may be able to take part if you*:
- Are at least 18 years of age
- Have a confirmed diagnosis of classic CAH due to 21-OH deficiency
- Take steroids daily (glucocorticoids with or without mineralocorticoids)
- Taking part is completely voluntary, and you may choose to stop at any time.
*Other criteria applies
What can I expect if I enroll?
Before the Study
Evaluations will be done (either at the clinic and/or at home) to see if you can take part in the trial.
During the Study
You will be chosen at random to receive either tildacerfont or a placebo (inactive pill). After the placebo period, everyone will receive tildacerfont. Visits and laboratory tests (blood and/or urine) will be done regularly during the study to monitor the safety of your treatment. Flexible visit options based on patient preferences include in-office, telemedicine, or home visits.
Travel reimbursement is available to support you in attending your study visits if needed. Please reach out to our study team to learn more about this option. Home health visits and home delivery of study drug may also be available, depending on what your site can offer.


Finding the support you need
The Spruce Patient Engagement Team
Living with a rare endocrine condition can be a complex journey you don’t have to navigate alone. The Spruce Patient Engagement Team offers personalized support and access to resources for people living with classic CAH.
Spruce Patient Engagement Liaisons (PELs) work closely with individuals, families, advocacy groups, and healthcare providers to better understand the needs of these communities. PELs provide education and resources related to these conditions, and serves as a contact resource for local advocacy and support groups.
“I am proud to serve as a trusted resource and
advocate for members of this community.”
– Mara VanAndel
Connecting with organizations and local support groups can help people understand more about their condition and meet others in the CAH community.





These groups and websites are neither owned nor controlled by Spruce Bio. Spruce does not endorse and is not responsible for the content or services they provide.
The purpose of the Spruce Patient Education Liaisons (PELs) is to provide education to patients, their families, and caregivers. PELs are employees of Spruce Bio. They are not acting as healthcare providers and are not part of your healthcare team. PELs do not provide medical care or advice. All diagnosis and treatment decisions should be made by you and your healthcare team.
Who qualifies for the CAHmelia Studies?
18 years of age and older and diagnosed with classic congenital adrenal hyperplasia.
Can I participate if I have non-classic CAH?
At this time, only individuals with classic CAH (including salt-wasting and simple virilizing) due to 21-hydroxylase deficiency are eligible for the CAHmelia studies.
Who is conducting the CAHmelia studies?
The CAHmelia studies are sponsored by Spruce Bio across 20 different countries, including the United States, Canada, Europe, South America, Asia and Australia.
Where will my study visits take place?
In certain circumstances, you can choose to have home health-care visits or telemedicine appointments instead of visits that would normally be in the clinic. For some tests, you will need to visit the clinic.
Will participants stop taking steroid treatment when starting tildacerfont?
Participants will NOT stop taking steroid treatment. Tildacerfont will not replace your steroid treatment but may allow you to manage your condition with lower doses of steroids.
What if I want to stop participating in the CAHmelia study?
Participation in CAHmelia studies are completely voluntary, you can freely withdraw (discontinue participation) at any time during the clinical trial.
What does it cost?
CAHmelia participants will receive CAHmelia study-related care, including medical tests, clinical care, stress-dosing steroids, and tildacerfont at no cost. Home health visits and home delivery of study drug may also be available, depending on what your site can offer.
References:1. Claahsen-van der Grinten HL, et al. Endocr Rev. 2022;43(1):91-159.doi: 10.1210/endrev/ bnab016; 2. Speiser PW, et al. J Clin Endocrinol Metab. 2018;103:4043–88; 3.Sarafoglou K,et al. J Clin Endocrinol Metab. 2021;106(11):e4666-e4679. doi:10.1210/clinem/dgab438;4. ClinicalTrials.gov. NCT04544410. Available at: https://clinicaltrials.gov/ct2/show/NCT04544410 (accessed May 23, 2022); 5. ClinicalTrials.gov.NCT04457336. Available at: https://clinicaltrials.gov/ct2/show/NCT04457336 (accessed May 23, 2022).

SPRUCE BIOSCIENCES, INC.
611 Gateway Blvd
Suite 740
South San Francisco, CA 94080 USA








