

Exploring Potential Solutions for Children and Adolescents Living with classic Congenital Adrenal Hyperplasia (CAH)
A clinical study evaluating tildacerfont in children and adolescents aged 2 to 17 with classic congenital adrenal hyperplasia (CAH)(1)
CAHptain Centers
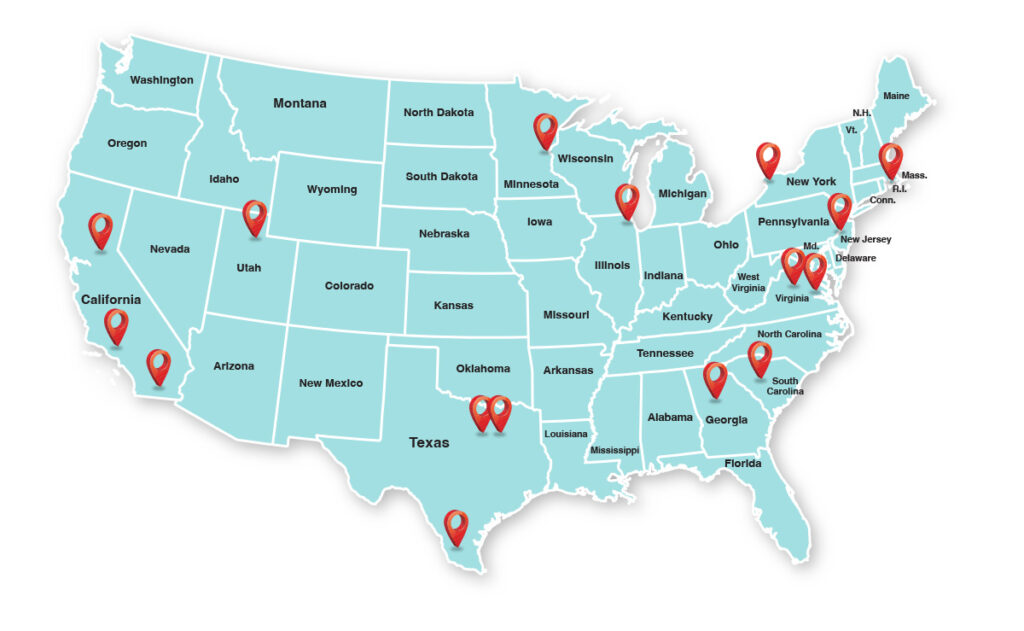
| State | City | Site/Institution |
| CA | Los Angeles | Children’s Hospital of Los Angeles |
| CA | Sacramento | Center of Excellence in Diabetes and Endocrinology (CEDE) |
| CA | San Diego | Rady Childrens Hospital San Diego – PIN |
| GA | Atlanta | Emory University |
| IL | Chicago | Ann and Robert H Lurie Childrens Hospital of Chicago |
| MN | Minneapolis | University of Minnesota |
| NY | Buffalo | UBMD |
| PA | Philadelphia | Children’s Hospital of Philadelphia |
| RI | Providence | Rhode Island Hospital |
| SC | Columbia | Prisma Health Midlands |
| TX | Dallas | Research Institute of Dallas |
| TX | Edinburg | DHR Health Institute for Research and Development |
| TX | Fort Worth | Cook Children’s Hospital |
| UT | Salt Lake City | University of Utah |
| VA | Charlottesville | University of Virginia |
| VA | Richmond | Virginia Commonwealth University |

Before a medication can be prescribed by a health care provider, it must be tested. Clinical trial programs are health-related research studies in humans that follow a pre-defined, detailed plan to determine the safety and effectiveness of the investigational medication for its intended use.
The primary purpose of the CAHptain study is to explore the safety and efficacy of tildacerfont in people with classic CAH ≥ 2 years of age through adulthood. Additionally, to evaluate if tildacerfont improves hormonal balance and allow participants to take lower doses of glucocorticoids.(1)
What is classic Congenital Adrenal Hyperplasia (CAH)?
Classic CAH is a genetic condition in both boys and girls that affects the body’s ability to create and regulate key hormones. In children with CAH, the enzyme (21-hydroxylase) needed to produce cortisol and aldosterone is not working properly. This hormone imbalance, as well as the glucocorticoid medication used to treat the imbalance, can cause problems with growth and development in children.
Managing CAH requires a delicate balance and can be challenging at times. Excess glucocorticoid can lead to stunted growth, weight gain and other long-term complications, such as hypertension and osteoporosis. Elevated androgen levels may occur from under treatment. As a result of over/under treatment:
- Children may experience early puberty and a narrow window for growth with impact on adult height.
- In adolescent girls, undertreatment may cause irregular periods and skin problems such as acne and excessive, male-pattern hair growth (hirsutism).
- In adolescent boys, poor CAH control can cause growth of testicular masses, called testicular adrenal rests (TARTs).(2)

To learn more about the condition of classic Congenital Adrenal Hyperplasia (CAH), watch these videos with SpruceBio Chief Medical Officer and Endocrinologist, Dr. Will Charlton.
Meet SpruceBio Chief Medical Officer, Dr. Will Charlton as he discusses classic Congenital Adrenal Hyperplasia (CAH) and it’s effects on patients.
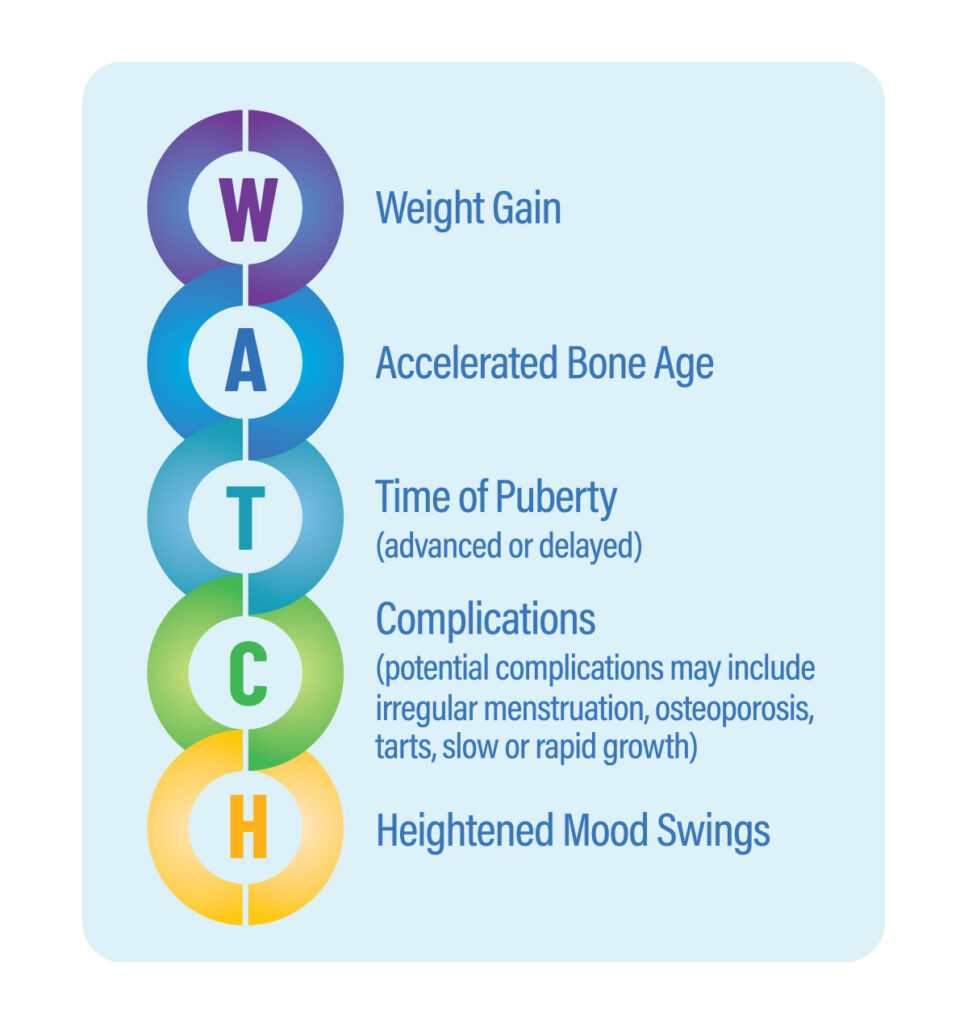
Signs and symptoms of over and under treatment to W.A.T.C.H
Weight Gain • Accelerated Bone Age • Time of Puberty (advanced or delayed) • Complications (potential complications may include irregular menstruation, osteoporosis, tarts, slow or rapid growth) • Heightened Mood Swings
Your body may be sending you a message about hormone imbalance from glucocorticoid (steroid) under-treatment or over-treatment.(3)
What is tildacerfont?
Tildacerfont is a new type of oral, once-daily investigational drug that is NOT a steroid.(4) By reducing the amount of androgens (testosterone-related hormones) your body makes, tildacerfont may improve your classic CAH symptoms.(4) This investigational drug will not replace your steroid treatment but may allow you to manage your condition with lower amounts of steroids.
Is tildacerfont safe?
Tildacerfont is generally well-tolerated in healthy volunteers and in people with classic CAH:
- Generally well-tolerated at doses under evaluation
- Generally well-tolerated across a diverse group of people
Tildacerfont is generally well-tolerated in healthy volunteers and people with classic CAH. Tildacerfont’s global history includes 12 studies with 320 participants, in 196 centers, and 26 countries.


Who can take part in this Study?
You may be able to take part if you*:
- Children and adults ≥ 2 years of age through adulthood
- Have a confirmed diagnosis of classic CAH due to 21-OH deficiency*
- Take steroids daily (glucocorticoids with or without mineralocorticoids)
- Taking part is completely voluntary, and you may choose to stop at any time.
*Other criteria applies
For more questions, ask your healthcare provider or site nearest to you.
What can I expect if I enroll?
Before the Study
Evaluations will be done at the clinic to see if you can take part in the trial.
During the Study
Everyone will receive tildacerfont tablets, to be taken daily. The tablets can be swallowed, crushed, or split. Visits and laboratory tests (blood and/or urine) will be done regularly during the study to monitor the safety of your treatment. The main part of the study is 4 weeks long but there is an optional extension where tildacerfont is given up to 92 more weeks.
The CAHptain study is dedicated to exploring potential solutions for children and adolescents living with classic CAH

Personalized Support for Patients and Families
Spruce Patient Engagement Team
The Spruce Patient Engagement Team offers personalized support and access to resources for families and patients diagnosed with classic CAH.
Spruce Patient Engagement Liaisons (PELs) serve as a partner, providing resources to help navigate each unique experience. PELs work closely with individuals, families, advocacy groups, and healthcare providers to support the needs of the CAH community.
“I am proud to serve as a trusted resource and
advocate for members of this community.”
– Mara VanAndel
Connecting with organizations and local support groups can help people understand more about their condition and meet others in the CAH community.





These groups and websites are neither owned nor controlled by Spruce Bio. Spruce does not endorse and is not responsible for the content or services they provide.
The purpose of the Spruce Patient Education Liaisons (PELs) is to provide education to patients, their families, and caregivers. PELs are employees of Spruce Bio. They are not acting as healthcare providers and are not part of your healthcare team. PELs do not provide medical care or advice. All diagnosis and treatment decisions should be made by you and your healthcare team.

FAQ’s
Who qualifies for the CAHptain Study?
Children and adults ≥ 2 years of age through adulthood with a confirmed diagnosis of classic congenital adrenal hyperplasia (CAH). Patients should be on a stable dose of glucocorticoids.
Will the CAHptain Study allow participation for non-classic CAH?
At this time, only individuals with classic CAH (including salt-wasting and simple virilizing) due to 21-hydroxylase deficiency are eligible for the CAHptain Study.
Who is conducting the CAHptain Study?
The CAHptain Study is sponsored by Spruce Biosciences in the US.
Where are the study locations?
Participants will be assigned to a study clinic as close to their home base as possible.
Will participants stop taking steroid treatment when starting tildacerfont?
Participants will NOT stop taking their steroid treatment. Tildacerfont will not replace steroid treatments, but may allow for the lowering of steroid dosing.
What if a participant decides that they want to stop participating in the CAHptain Study?
Participation in the study is completely voluntary and participants are free to withdraw from the study at any time during the clinical trial.
What does it cost to participate in the CAHptain Study?
References:
1. https://clinicaltrials.gov/ct2/show/NCT05128942; 2. Speiser PW, et al. J Clin Endocrinol Metab. 2018;103:4043–88; 3. Claahsen-van der Grinten HL, et al. Endocr Rev. 2022;43(1):91-159.doi: 10.1210/endrev/ bnab016; 4. Sarafoglou K,et al. J Clin Endocrinol Metab. 2021;106(11):e4666-e4679. doi:10.1210/clinem/dgab438

SPRUCE BIOSCIENCES, INC.
611 Gateway Blvd
Suite 740
South San Francisco, CA 94080 USA







